What Does the Second Quantum Number L Describe
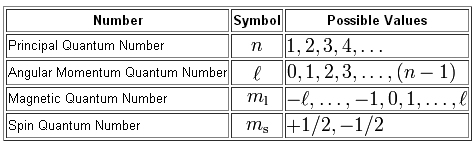
The four quantum numbers used to describe the electrons are n2 ℓ1 m1 0 or -1 and s12 the electrons have parallel spins. The azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron.
This signifies the energy level within a subshell.

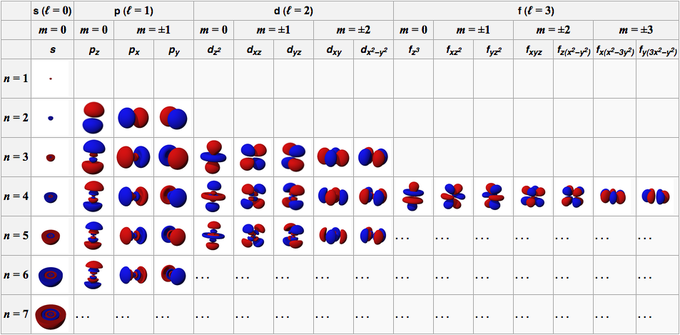
. Corresponds to spd and f subshells containing spdf orbitals respectively. It signifies the shape or type of the orbital. In chemistry and spectroscopy ℓ 0 is called an s orbital ℓ.
The angular momentum quantum number l also referred to as the secondary quantum number or azimuthal quantum number describes the shape of the orbital that an electron occupies. N principle quantum number 2 l angular momentum quantum number 0 ml magnetic quantum number 0 ms spin quantum number 12 or -12 Possible values of the magnetic quantum number m. L 2 ħ 2 ℓ ℓ 1 In chemistry and spectroscopy ℓ 0 is called s orbital ℓ 1 p orbital ℓ 2 d orbital and ℓ 3 f orbital.
The second quantum number is the angular quantum number ℓ. L 1 l where Lz is the z-component of the angular momentum and ml is the angular momentum projection quantum number. The first of these is n the principal quantum number and it is restricted to integer values 1 2 3.
For example the n 3 shell has subshells of l 012 which means the n 3 shell contains s p and d subshells each containing their respective orbitals. The third quantum number or magnetic quantum number is designated by. N principle quantum number 2 l angular momentum quantum number 0 ml magnetic quantum number 0 ms spin quantum number 12 or -12 Possible values of the magnetic quantum number m.
The angular momentum quantum number l describes the shape of the subshell and its orbitals where l 0123. The angular quantum number l describes the shape of the orbital. There are four quantum numbers namely principal azimuthal magnetic and spin quantum numbers.
Quantum Numbers Principal Azimuthal Magnetic and Spin - The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. The azimuthal quantum number also known as the angular momentum quantum number or orbital quantum number describes the subshell and gives the magnitude of the orbital angular momentum through the relation. The second quantum number is l the orbital angular momentum quantum number and it must also be an integer such that it can be at most n 1.
It is also known as the orbital angular momentum quantum number orbital quantum. To learn Detailed Explanation of Different Types of Quantum Numbers Visit BYJUS for more. The second quantum number or orbital angular momentum quantum number is designated by the letter l.
The rule in parentheses for the values of ml is that it can range from l to l in steps of one. Second Quantum Number. The angular momentum quantum number l is an integer that has possible values of 0 1 2 3 etc.
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital. Not Just for Electrons While quantum numbers are commonly used to describe electrons they may be used to describe the nucleons protons and neutrons of an atom or elementary particles. Up to 10 cash back It signifies the electron shell or the energy level of the electron.
They can even take on more complex shapes as the value of the angular quantum number becomes larger. Lz ml h 2π ml ll1101l1l L z m l h 2 π m l l l 1 1 0 1. Each value of n has multiple values of ℓ ranging in values from 0 to n-1This quantum number determines the shape of the electron cloud.
Orbitals have shapes that are best described as spherical l 0 polar l 1 or cloverleaf l 2. It can take values from 0 to n-1 where n is the principal quantum number. The second quantum number known as the angular or orbital quantum number describes the subshell and gives the magnitude of the orbital angular momentum through the relation.
The angular momentum quantum number determines the shape of the orbital.

Quantum Numbers Free Textbooks Khan Academy Never Stop Learning

No comments for "What Does the Second Quantum Number L Describe"
Post a Comment